Radius Health, Inc
.
RDUS
recently announced that the first patient has been randomized in phase II/III study, SCOUT-015, of pipeline candidate, RAD011.
SCOUT-015 is a global, randomized, double-blind, placebo-controlled phase II/III study evaluating RAD011, a synthetic cannabidiol oral solution, for treating hyperphagia and related neuro-behavioral symptoms in Prader-Willi Syndrome (PWS).
The study’s design allows for evaluating multiple-dose groups and Intent-To-Treat efficacy analysis with both phase II and phase III cohorts.
As of now, nine sites are activated in the United States for screening, and patient recruitment will continue across the United States and globally as non-U.S. sites get activated.
Please note that RAD011 enjoys Orphan Drug status in the United States. The experimental candidate has also been granted Fast Track by the FDA.
PWS is a rare neuro-endocrine orphan disease that affects 20,000 to 30,000 patients in the United States.
Shares of RDUS have gained 48% in the year so far against the
industry
’s decline of 19.5%.
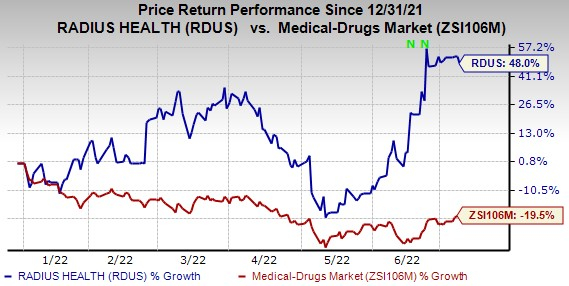
Image Source: Zacks Investment Research
Last month, Radius Health announced that it will cease all work on abaloparatide transdermal system (abalo-TDS) development program. The company had earlier outlined three requirements needed to move the abalo-TDS program forward. These were regulatory clarity, a re-constructed supply chain and CMC agreement/economics and external funding.
However, a response from the FDA indicated that an additional pivotal trial will be required to move forward with any regulatory filing.
Hence, given the clarity on the regulatory pathway, delayed commercial timelines and more than $100 million of additional capital required over the next three years, Radius Health will cease all work on abalo-TDS.
The company is also going private. RDUS has entered into a definitive agreement to be acquired by Gurnet Point Capital and Patient Square Capital for a total consideration of $890 million. The price of $890 million includes the assumption of debt and full payment of the CVR (Contingent Value Right).
Radius Health’s lead drug Tymlos approved for osteoporosis is facing challenges.
Competition is stiff for Tymlos from
Eli Lilly & Co’s
LLY
Forteo and
Amgen
‘s
AMGN
Prolia.
Eli Lilly’s Forteo generated sales of $137 million in the first quarter.
Amgen’s Prolia sales increased 12% year over year in the March quarter, driven by 10% volume growth and a higher net selling price. AMGN’s Evenity is also approved for treating osteoporosis in postmenopausal women, who are at high risk of fracture or cannot use another osteoporosis medicine or other osteoporosis medicines did not work well.
Radius Health currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the healthcare sector is
Geron
GERN
, which at present carries a Zacks Rank #2 (Buy). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
GERN’s loss estimates for 2022 have narrowed by 6 cents in the past 60 days. Geron surpassed on earnings in three of the trailing four quarters and missed the mark in the remaining one, the average surprise being 1.07%.
Just Released: Zacks Top 10 Stocks for 2022
In addition to the investment ideas discussed above, would you like to know about our 10 top picks for the entirety of 2022?
From inception in 2012 through 2021, the
Zacks Top 10 Stocks
portfolios gained an impressive +1,001.2% versus the S&P 500’s +348.7%. Now our Director of Research has combed through 4,000 companies covered by the Zacks Rank and has handpicked the best 10 tickers to buy and hold. Don’t miss your chance to get in…because the sooner you do, the more upside you stand to grab.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








