Shares of
Rigel Pharmaceuticals, Inc
.
RIGL
plummeted 60.2% after the company announced that its late-stage study of fostamatinib was not successful.
The phase III clinical trial, a global, multi-center, randomized, double-blind, placebo-controlled trial, FORWARD, is evaluating fostamatinib in patients with warm autoimmune hemolytic anemia (wAIHA).
However, the study did not demonstrate statistical significance in the primary efficacy endpoint of durable hemoglobin response in the overall study population.
Patients treated with fostamatinib had a favorable durable hemoglobin response compared to placebo as shown in a post-hoc regional analysis of U.S., Canadian, Australian and Western European trial sites. However, in the Eastern European trial sites, patients did not show any such response.
Of the 90 patients that completed the FORWARD phase III study, 71 (79%) enrolled in the open-label extension study. Data from this study will be reported later.
We note that fostamatinib is already approved in the United States under the brand name Tavalisse for the treatment of adult patients with chronic immune thrombocytopenia who have had an insufficient response to a previous treatment.
The drug generated sales of $16.2 million in the first quarter.
The successful development of the drug for another indication would have been a great boost for the company. The dismal results disappointed investors.
Shares of Rigel have plunged 75% so far this year compared with the
industry
’s decline of 22.2%.
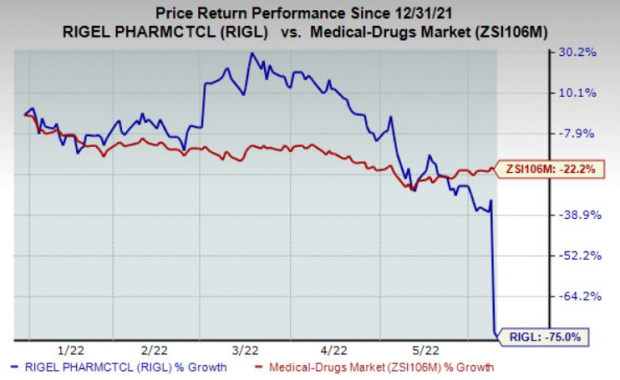
Image Source: Zacks Investment Research
Fostamatinib is also currently being studied in a phase III study for the treatment of hospitalized high-risk patients with COVID-19 and an NIH/NHLBI-sponsored phase III study for the treatment of COVID-19 in hospitalized patients.
Rigel’s other clinical programs include its interleukin receptor-associated kinase (IRAK) inhibitor program and a receptor-interacting serine/threonine-protein kinase (RIPK) inhibitor program in clinical development with partner
Eli Lilly and Company
LLY
.
Eli Lilly continues to advance R552, and the initial phase II study in an immunologic disease indication is now anticipated to begin in the first quarter of 2023.
Rigel currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks are
Alkermes
ALKS
and
Geron Corporation
GERN
, both carrying a Zacks Rank #2 (Buy). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
ALKS’ loss estimates for 2022 have narrowed to 3 cents from a loss of 14 cents in the past 60 days. Alkermes surpassed earnings in all the trailing four quarters, the average being 350.48%.
GERN’s loss estimates for 2022 have narrowed 6 cents in the past 60 days. Geron surpassed on earnings in three of the trailing four quarters and missed the mark in one, the average surprise being 1.07%.
Profiting from the Metaverse, The 3rd Internet Boom (Free Report):
Get Zacks’ special report revealing top profit plays for the internet’s next evolution. Early investors still have time to get in near the “ground floor” of this $30 trillion opportunity. You’ll discover 5 surprising stocks to help you cash in.
Download the report FREE today >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








