Roche
RHHBY
announced that the FDA has approved Actemra (tocilizumab) intravenous (IV) for the treatment of COVID-19 in hospitalized adult patients who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO).
The approval comes on the heels of yet another surge in COVID-19 cases across the globe, attributed to emerging Omicron sub-variants.
In June 2021, the FDA granted Emergency Use Authorization (EUA) to Actemra for use in hospitalized adults and children (aged 2 and older) with COVID-19. The use of Actemra to treat hospitalized people aged 2 to less than 18 years old is not FDA-approved, but the EUA for this age group currently remains in place after the FDA’s approval for hospitalized adult patients.
Per the company, Actemra is the first FDA-approved monoclonal antibody to treat COVID-19 and is recommended for use as a single 60-minute IV infusion.
The FDA approval is based on the results of the RECOVERY trial as well as the EMPACTA study, the first global, phase III study in COVID-19 to focus on patients from underrepresented racial and ethnic groups. Actemra was evaluated for the treatment of COVID-19 in four randomized, controlled studies involving more than 5,500 hospitalized patients. The results of these four studies (the University of Oxford-led RECOVERY trial along with the Roche-sponsored global trials EMPACTA, COVACTA and REMDACTA) showed that Actemra may improve outcomes in patients receiving corticosteroids and requiring supplemental oxygen or breathing support.
Actemra is approved for use in more than 30 countries for this indication. This is the seventh FDA-approved indication for the drug in the United States since the drug was launched in 2010.
Actemra/RoActemra, an interleukin-6 (IL-6) receptor antagonist, is approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis (RA) who have used one or more disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX), that did not provide enough relief. The subcutaneous injection is also approved for the treatment of adult patients with giant cell arteritis (GCA), for the treatment of patients two years of age and older with active polyarticular juvenile idiopathic arthritis (PJIA) or active systemic juvenile idiopathic arthritis (SJIA), and for slowing the rate of decline in pulmonary function in adult patients with systemic sclerosis-associated interstitial lung disease (SSc-ILD).
Shares of Roche have declined 22.8% in the year so far against the
industry
’s increase of 12.1%.
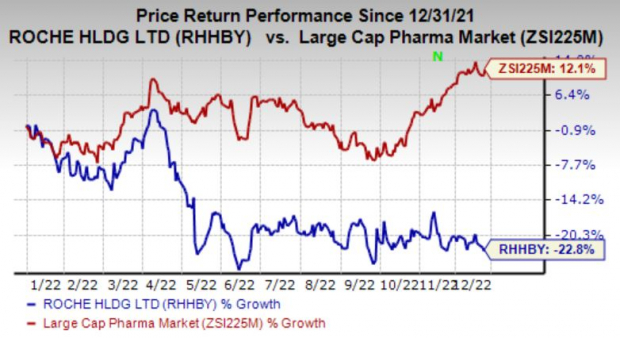
Image Source: Zacks Investment Research
Strong demand for new drugs, namely Hemlibra, Ocrevus (multiple sclerosis), Evrysdi (spinal muscular atrophy), Phesgo (cancer) and Tecentriq (cancer), maintains momentum for the company and offsets stiff competition from biosimilars for some of Roche’s key drugs, such as Avastin, MabThera/Rituxan and Herceptin.
Last month, the FDA announced that
Eli Lilly’s
LLY
bebtelovimab is not currently authorized for emergency use in the United States because it is not expected to neutralize Omicron subvariants BQ.1 and BQ.1.1., which were the predominant subvariants in the country.
Based on pseudovirus data, Lilly stated that it can confirm that bebtelovimab does not retain neutralization activity against the BQ.1 and BQ.1.1 variants, most likely due to an amino acid K444T substitution. Hence, Lilly and the FDA agreed that it is not medically appropriate to treat high-risk patients with mild-to-moderate COVID-19 with bebtelovimab in the United States at this time.
Zacks Rank and Key Picks
Roche currently carries a Zacks Rank #3 (Hold). A couple of better-ranked stocks in the overall healthcare sector are
Sanofi
SNY
and
Gilead Sciences, Inc.
GILD
, both carrying a Zacks Rank #2 (Buy). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Earnings estimates for Sanofi have increased 17 cents in the last 90 days to $4.27. Sanofi has surpassed earnings estimates in all of the past four quarters with an average beat of 9.50%.
Earnings estimates for Gilead Sciences have increased 48 cents in the last 60 days to $7.08. Gilead has surpassed earnings estimates in three of the past four quarters with an average beat of 0.36%.
Zacks Top 10 Stocks for 2023
In addition to the investment ideas discussed above, would you like to know about our 10 top picks for the entirety of 2023? From inception in 2012 through November, the
Zacks Top 10 Stocks
portfolio has tripled the market, gaining an impressive +884.5% versus the S&P 500’s +287.4%.
Now our Director of Research is combing through 4,000 companies covered by the Zacks Rank to handpick the best 10 tickers to buy and hold. Don’t miss your chance to get in on these stocks when they’re released on January 3.
Be First to New Top 10 Stocks >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








