Roche
RHHBY
recently announced that the European Commission has granted conditional marketing authorization for the CD20xCD3 T-cell engaging bispecific antibody Lunsumio (mosunetuzumab).
The drug has been approved for treating adult patients with relapsed or refractory (R/R) follicular lymphoma (FL) who have received at least two prior systemic therapies.
Lunsumio is a first-in-class CD20xCD3 T-cell engaging bispecific antibody designed to target CD20 on the surface of B-cells and CD3 on the surface of T-cells.
The approval is based on positive results from the phase I/II GO29781 study, wherein Lunsumio demonstrated high complete response rates, with the majority of complete responders maintaining responses for at least 18 months and favorable tolerability in people with heavily pre-treated FL.
After a median follow-up of 18.3 months, the median duration of response among responders was 22.8 months, the complete response rate was 60% and the objective response rate was 80%.
We note that conditional approval is granted to a drug that fulfills an unmet medical need wherein the benefit of immediate availability outweighs the risk of less comprehensive data than normally required.
Roche is currently evaluating Lunsumio in two phase III studies: CELESTIMO, investigating Lunsumio plus Revlimid in second-line plus FL, and SUNMO, investigating Lunsumio plus Polivy (polatuzumab vedotin) in 2L+ diffuse large B-cell lymphoma (DLBCL).
Earlier, Polivy was approved in combination with MabThera (rituximab) plus cyclophosphamide, doxorubicin and prednisone (R-CHP) in previously untreated DLBCL.
The approval of additional drugs will bolster Roche’s hematology portfolio, which includes MabThera, Gazyvaro (obinutuzumab), Polivy (polatuzumab vedotin), Venclyxto (venetoclax) in collaboration with Hemlibra (emicizumab).
Concurrently, the European Commission also approved Tecentriq (atezolizumab) as an adjuvant treatment, following complete resection and platinum-based chemotherapy, for adults with non-small cell lung cancer (NSCLC) with a high risk of recurrence whose tumors express PD-L1≥50% and who do not have EGFR mutant or ALK-positive NSCLC.
This approval was based on the phase III IMpower010 study, wherein adjuvant Tecentriq reduced the risk of disease recurrence or death by 57% in people with PD-L1 high resected Stage II-III NSCLC compared with best supportive care.
Tecentriq is approved as a first-line treatment for adults with extensive-stage small-cell lung cancer (SCLC) in combination with carboplatin and etoposide (chemotherapy). Tecentriq also has four approved indications in advanced or metastatic NSCLC as either a single agent or in combination with targeted therapies and/or chemotherapies.
However, Tecentriq faces stiff competition from
Merck
’s
MRK
Keytruda and
Bristol Myers’
BMY
Opdivo, which are also approved for similar indications.
Approved for various oncology indications, Keytruda is Merck’s key driver.
Opdivo is one of the top revenue generators for Bristol Myers and the continued label expansion of the drug for additional indications should further boost growth. In particular, demand for the drug to treat first-line lung, renal and gastric cancer as well as adjuvant esophageal and bladder cancers boosted its growth.
Roche’s stock has lost 21.4% in the year so far against the
industry
’s growth of 5%.
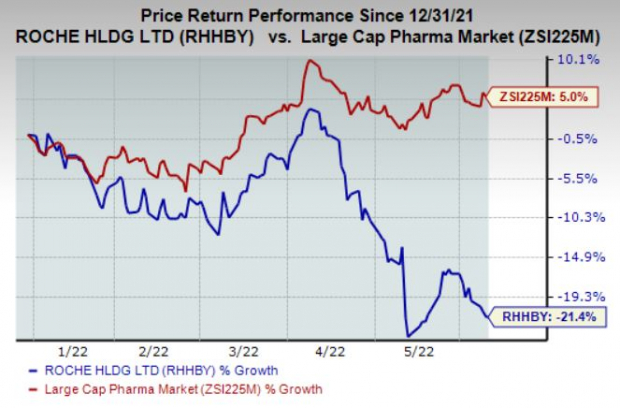
Image Source: Zacks Investment Research
Roche has a solid and broad oncology portfolio, and approval of additional drugs or label expansion of existing drugs will further bolster it.
Its performance in the March quarter was encouraging on the back of its diagnostics division, which maintained its stellar performance on demand for conducting COVID-19 tests. The pharmaceuticals business was also stable and newer drugs continued to offset the decline in sales of the legacy drugs.
However, the outlook indicated that sales will decline in 2022 from the prior-year reported figure due to reduced demand for COVID-19 medicines and diagnostics.
Roche currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Profiting from the Metaverse, The 3rd Internet Boom (Free Report):
Get Zacks’ special report revealing top profit plays for the internet’s next evolution. Early investors still have time to get in near the “ground floor” of this $30 trillion opportunity. You’ll discover 5 surprising stocks to help you cash in.
Download the report FREE today >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








