Sanofi
SNY
announced that the European Commission granted marketing approval to two new enzyme replacement therapies, Nexviadyme (avalglucosidase alfa) and Xenpozyme (olipudase alfa).
Nexviadyme, an enzyme replacement therapy, is approved for the treatment of Pompe disease, a rare degenerative muscle disorder. Nexviadyme is approved for both late-onset Pompe disease and infantile-onset Pompe disease and is the first new drug approved for Pompe disease in Europe in more than 15 years. The last Pompe disease drug approved in Europe was Sanofi’s drug, Myozyme.
Avalglucosidase alfa was approved in the United States in August 2021 in the name of Nexviazyme. Nexviazyme recorded sales of €30 million in the first quarter of 2022. Nexviazyme is also approved in Japan, Canada, Switzerland, Australia, Brazil, Taiwan and the United Arab Emirates.
Sanofi’s stock has risen 5% this year so far compared with the
industry
’s increase of 5.9%.
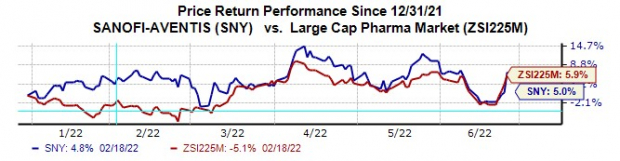
Image Source: Zacks Investment Research
Nexviadyme’s approval was based on data from two pivotal studies, which include both infantile-onset and late-onset Pompe disease patients.
Pompe disease causes a debilitating deterioration of the muscles resulting in decreased respiratory function and mobility in affected patients. Nexviazyme targets the M6P receptor, the key pathway for cellular uptake of enzyme replacement therapy. In a phase III study called COMET, Nexviazyme resulted in improvements in respiratory function and walking distance in patients with LOPD.
In November 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency recommended approving avalglucosidase alfa. However, the CHMP considered that avalglucosidase alfa does not qualify as a New Active Substance (“NAS”), which means a substance not previously authorized as a medicinal product in the EU. In April 2022, the Committee for Orphan Medicinal Product (COMP) also recommended the removal of Nexviadyme from the Community Register of Orphan Medicinal Products (OMP).
In a separate press release, Sanofi announced that Xenpozyme (olipudase alfa) was approved for the treatment of adult and pediatric patients with non-Central Nervous System (CNS) manifestations of acid sphingomyelinase deficiency (ASMD), a rare, progressive, and potentially life-threatening genetic disease in Europe. It is the first and only disease-specific therapy for ASMD in Europe.
Xenpozyme is already approved in Japan for ASMD. An application seeing approval for olipudase alfa is under review in the United States, with a decision expected next week
Xenpozyme’s approval was based on data from two clinical studies, in which Xenpozyme led to robust and clinically relevant improvement in lung function and reduced spleen and liver volumes. Enlarged spleen or liver, difficulty in breathing, lung infections, and unusual bruising or bleeding are some of the signs and symptoms of this rare disease, which particularly affects infants and children and is associated with substantial morbidity and risk of premature death.
Nexviadyme, on approval, could face potential competition from
Amicus Therapeutics
’
FOLD
AT-GAA for the treatment of Pompe disease. The candidate consists of two components — cipaglucosidase alfa — an engineered enzyme to enhance lysosomal uptake into cells and miglustat, a stabilizer of the engineered enzyme. The FDA has accepted Amicus Therapeutics’ two regulatory filings for AT-GAA — a biologics license application (BLA) for cipaglucosidase alfa and a new drug application (NDA) for miglustat – for Pompe disease. The FDA has set PDUFA action dates of Aug 29, 2022 for the NDA and Oct 29, 2022 for the BLA. Amicus Therapeutics’ marketing authorization application submissions for AT-GAA with the European Medicines Agency were filed and validated in the fourth quarter of 2021.
Sanofi currently has a Zacks Rank #4 (Sell). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Stocks to Consider
Some better-ranked biotech stocks are
Novo
Nordisk
NVO
and
Sesen Bio
SESN
. While Sesen Bio sports a Zacks Rank of 1, Novo Nordisk has a Zacks Rank of 2 (Buy). You can see
the complete list of today’s Zacks #1 Rank stocks here
.
Novo Nordisk’s earnings per share have risen from $3.35 per share to $3.48 per share for 2022 and from $3.94 per share to $4.19 per share over the past 60 days.
Earnings of Novo Nordisk beat estimates in each of the last four quarters, the average surprise being 7.56%. The stock has risen 2.5% in the past three months.
The Zacks Consensus Estimate for Sesen Bio’s2022 loss has declined from 46 cents to 44 cents per share in the past 60 days. Shares of SESN have risen 20.6% in the past three months.
Earnings of Sesen Bio beat estimates in three of the last four quarters and missed the mark on one occasion, the average surprise being 69.9%.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report








