Sanofi
SNY
and partner
Regeneron
REGN
announced that the FDA has accepted their supplemental Biologics License Application (BLA) seeking label expansion of their blockbuster medicine, Dupixent (dupilumab) for treating adult patients with uncontrolled prurigo nodularis, a chronic inflammatory skin disease. The FDA has also granted priority review to the NDA, shortening its review period from the standard 12 months to 8 months.
The sBLA is supported by data from two pivotal studies — PRIME and PRIME2 — which evaluated efficacy and safety of Dupixent in adult patients with prurigo nodularis.
The top-line data from the PRIME study showed that 60% of the patients treated with Dupixent experienced a clinically meaningful reduction in itch from baseline compared with 18% of the patients in the placebo arm — the primary endpoint. Also, 48% of the patients treated with Dupixent achieved clear or almost clear skin compared to 18% of placebo patients at week 24.
The top-line data from the phase III PRIME2 study showed that 37% of the patients who were treated with Dupixent experienced a clinically meaningful reduction in itch from baseline as compared to 22% of patients in the placebo arm — the primary endpoint. The data also showed that 58% of patients who received Dupixent experienced a clinically meaningful reduction in itch from baseline versus 20% of patients who received placebo at week 24 of treatment. Also, 45% of the patients who were treated with Dupixent achieved clear or almost clear skin compared to 16% of placebo patients at week 24.
Data from the studies demonstrates that Dupixent has the potential to significantly improve disease signs and symptoms compared to placebo, including a reduction in itch and skin lesions.
A decision from the FDA regarding the approval of the label expansion of the drug to include adult prurigo nodularis patients is expected in September. A potential approval can be much earlier than September as the FDA approved a label expansion of the drug to include eosinophilic esophagitis patients within one and a half months of acceptance of the sBLA for the same. Dupixent is now the first and only medicine indicated for eosinophilic esophagitis in the United States, following its approval earlier this month.
Prurigo nodularis will be the fifth FDA-approved disease indication for Dupixent following a potential approval. Sanofi and Regeneron’s Dupixent is already approved in the United States, EU and some other countries for three type II inflammatory diseases, namely for certain patients with chronic rhinosinusitis with nasal polyposis, asthma and atopic dermatitis in different age populations. Dupixent is under review in Europe for eosinophilic esophagitis.
Sanofi stock has risen 10.1% this year so far compared with the
industry
’s increase of 6.5%.
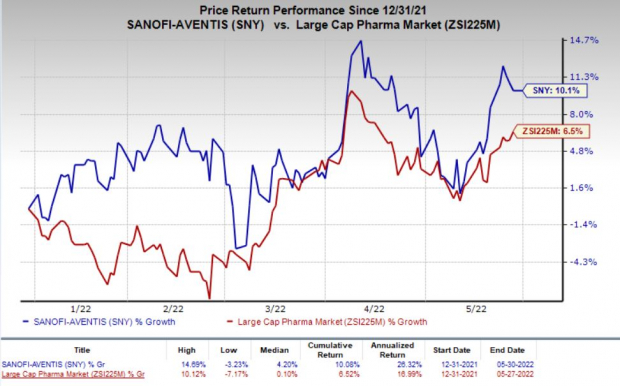
Image Source: Zacks Investment Research
Dupixent is being jointly marketed by Regeneron and Sanofi under a global collaboration agreement. Sanofi records global net product sales of Dupixent while Regeneron records its share of profits/losses in connection with the global sales of the drug.
Dupixent sales are now annualizing at close to €6.0 billion after around three years on the market. Sanofi expects Dupixent to achieve more than €13 billion in peak sales. The drug’s frequent label expansion approvals are driving sales higher. With outside U.S. revenues accelerating and multiple approvals for new indications and expansion in younger patient populations expected in the near future, its sales are expected to be higher.
Sanofi and Regeneron are also studying dupilumab in late-stage studies in a broad range of diseases, driven by type II inflammation like bullous pemphigoid, chronic spontaneous urticaria, prurigo nodularis, and chronic obstructive pulmonary disease.
Zacks Rank & Stocks to Consider
Sanofi currently has a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
A couple of better-ranked biotech stocks are
Sesen Bio
SESN
and
Alkermes
ALKS
. While Sesen Bio sports a Zacks Rank #1, Alkermes carries a Zacks Rank #2 (Buy).
The Zacks Consensus Estimate for Sesen Bio’s 2022 loss has narrowed from 33 cents to 31 cents per share in the past 60 days. Shares of SESN have declined 35.6% in the year-to-date period.
Earnings of Sesen Bio beat estimates in three of the last four quarters and missed the mark on one occasion, the average surprise being 69.94%.
The Zacks Consensus Estimate for Alkermes’ 2022 loss per share has narrowed from 69 cents to 60 cents in the past 60 days. Shares of ALKS have risen 31.3% year to date.
Earnings of Alkermes beat estimates in each of the last four quarters, the average being 362.62%.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
It’s a little-known chemical company that’s up 65% over last year, yet still dirt cheap. With unrelenting demand, soaring 2022 earnings estimates, and $1.5 billion for repurchasing shares, retail investors could jump in at any time.
This company could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in little more than 9 months and NVIDIA which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









