When lithium-ion batteries were first introduced, they took the market by storm. These batteries have high energy capacity which allows them to power more than just the apps running on your phone. However, many speculate that the current lithium-ion battery technology is on it’s last leg and the venture for a better lithium-battery is underway. But before this is possible, academics need to find a way of maneuvering around the problem of dendrites. Scientists at Rice University in Texas have developed a new technology and if all goes as planned, it could help to solve this problem by creating lithium-metal batteries that hold three times the energy that lithium-ion batteries hold.
So how does a lithium-ion battery work? Well, there is an anode on one side and an electrode on the other side, and dendrites, which are tiny lithium fibers, will form on the anodes during charging and then spread until they reach the electrode, thus causing the battery to short circuit and fail. As a result, this short circuit can cause lithium-ion batteries to catch fire or explode, and companies like Samsung learnt this the hard way.
James Tour, the lead researcher of the study at Rice University, stated, “Lithium-ion batteries have changed the world, no doubt. But they’re about as good as they’re going to get. Your cellphone’s battery won’t last any longer until new technology comes along.”
Dendrites causing lithium-ion batteries to short circuit is one of the main problems seen with these batteries and until scientists can create a technique to solve this issue, we just have to hope that a higher capacity and faster-charging battery is underway. With that said, scientists haven’t just ignored the issue, there have been a number of attempts to solve the dendrite problem, including using Kevlar nanofibers to slow down the growth of dendrites and producing a new electrolyte which could lead to an anode-free cell. So, where does the new technology from Rice University fit into the equation?
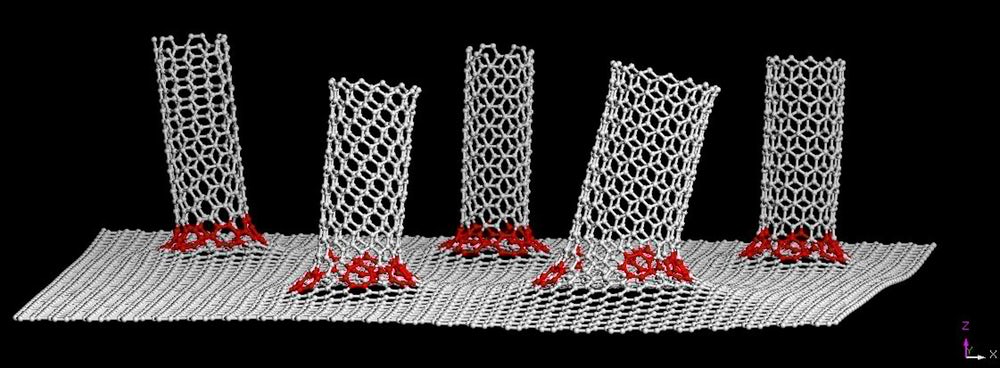
First and foremost, the new technology will be able to stop the growth of dendrites dead in it’s tracks. How is that possible? The key is an anode made from a material that was first manufactured at Rice University five years ago. Using a covalent bond structure, it integrates a 2D graphene sheet and carbon nanotubes to create a seamless 3D structure. When the material was first announced, Tour stated:
“By growing graphene on metal (in this case copper) and then growing nanotubes from the graphene, the electrical contact between the nanotubes and the metal electrode is ohmic. That means electrons see no difference, because it’s all one seamless material.”
When the material was initially introduced, it was only meant to be used for energy storage and electronics applications like supercapacitors. That was until 2014 when Abdul-Rahman Raji, the co-lead author of the study, started to play around with lithium metal and the graphene-nanotube hybrid. It was then that Raji noticed that the material had the potential to act as a dendrite inhibitor.
Following the discovery, Raji stated, “I reasoned that lithium metal must have plated on the electrode while analyzing results of experiments carried out to store lithium ions in the anode material combined with a lithium cobalt oxide cathode in a full cell.” He further noted, “We were excited because the voltage profile of the full cell was very flat. At that moment, we knew we had found something special.”
Taking a closer look at the finding, it was revealed that there were no dendrites growing after the lithium metal was placed into a standalone hybrid anode. But the question that remains is, will this work in a traditional battery?
To put the anode to the test, the team of researchers created full battery prototypes with sulfur-based cathodes which retained 80% capacity after roughly 500 charge-discharge cycles. This is equal to what a cellphone goes through in a two-year time frame. Researchers found that there were no dendrites on the anodes.
So how does this work?
Essentially the low density and high surface area of the nanotube will allow for the lithium metal to cover the carbon hybrid material evenly when the battery is going through a charging cycle. Plus, there is an abundance of room for the particles to slip in and out during both the charge and discharge cycle. Therefore, it ends up being evenly spread out which halts the overall growth of dendrites.
As reported by Rice University’s study, the anode material is able to have a lithium storage capacity of 3,351 milliamp hours per gram. This is verging pure lithium’s hypothetical maximum of 3,860 milliamp hours per gram and 10 times that of lithium-ion batteries. Additionally, the nanotube carpet has a low density, thus allowing it to cover all the way down to surface and utilize use of the available volume.
“Many people doing battery research only make the anode, because to do the whole package is much harder,” stated Tour. “We had to develop a commensurate cathode technology based upon sulfur to accommodate these ultrahigh-capacity lithium anodes in first-generation systems. We’re producing these full batteries, cathode plus anode, on a pilot scale, and they’re being tested.”
Featured Image: Phys.org/Tour Group/Rice University