Biogen (NASDAQ:BIIB) and Eisai Co., Ltd (TYO:4523) has just had their Alzheimer’s disease drug Aduhelm approved by the FDA on June 7, 2021. Investors holding the stock on Friday June 4 had their shares close at $286.14, and finished off Monday afternoon on June 7 at $395.85 per share. With such a massive increase in share price in one day, it’s one of the most lucrative FDA approvals of the year. Since June 4, the stock is up 33%. It was a big win for investors in a situation where many believed the FDA approval for Aduhelm would not go ahead.

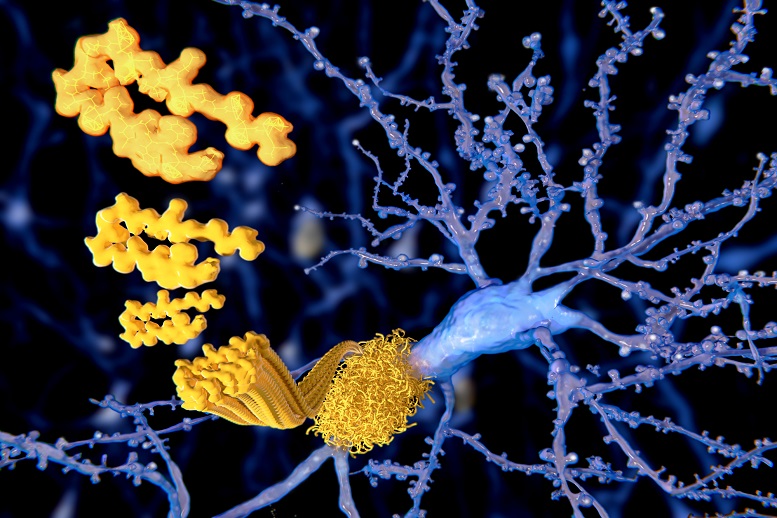
The company announced the US Food and Drug Administration (FDA) provided accelerated approval for Aduhelm, which reduces amyloid beta plaques in the brain according to this release. Data from initial clinical trials allowed for accelerated approval because the drug demonstrated the effect of reducing amyloid beta plaques. These are peptides that latch on to neurons, causing the degeneration that results in Alzheimer’s and dementia.
Also, Biogen (NASDAQ:BIIB) is not the only stock to rise since FDA approval of Aduhelm.
At this time, about 1000 treatment sites (clinics, hospitals and care homes) are prepared to receive the drug. This is being called by analysts as ‘one of the biggest drug launches in biopharma history.’ Biogen (NASDAQ:BIIB) may hit US$10 billion from the sales of Aduhelm according to said article.
In terms of function, Aduhelm (aducanumab) destroys amyloid plaques that stick to neurons causing degeneration. According to the FDA, reducing amyloid plaques is ‘reasonably likely to predict important benefits to patients.’ Alzheimer’s affects over 6 million Americans, and estimates say it will affect 12.7 million by 2050.
The One Problem? The Price per Patient
While FDA approval is one thing for one of the most promising Alzheimer’s drugs to date, it’s important to say that the drug is not yet proven to slow the disease. It’s scored well in trials, and only the next several years will determine whether or not the drug ‘works.’
Patients are going to be the hardest hit financially – those who wish to be approved for the new treatment can expect a cost of approximately US$56,000. It’s understandable if this doesn’t deter some patients, as the last time the FDA approved an Alzheimer’s treatment was in 2003. At a price point of $4,312 per treatment, many advocates are furious. At the end of the day, it’s the patients who are most vulnerable, and the most affected.
When Gregory Guercio’s wife Marie was diagnosed with Alzheimer’s at 72, over two years ago, they had not had much luck with treatments. Guercio now hopes that Aduhelm can provide better results. “When something like this comes out, you reach out for anything… You don’t have any expectations, you just hope.”
Featured image from Megapixl