Valneva
’s
VALN
shares increased 9.2% on Mar 8, after the company announced that it has successfully completed a pivotal phase III study evaluating its single-shot chikungunya vaccine candidate, VLA1553, in patients aged 18 years and older.
The phase III study achieved all primary and secondary endpoints. Data from the study demonstrated a very high level of seroprotection — 98.9% of participants achieved protective levels of chikungunya virus (CHIKV) neutralizing antibodies after one month of vaccine administration. The study also showed that 96.3% of the participants achieved a protective level of CHIKV neutralizing antibodies after six months of dose administration.
The vaccine also achieved high levels of seroprotection in elderly participants aged 65 years and above, which were similar to the levels achieved in younger adult participants.
The levels of seroprotection achieved by VLA1553 have crossed the 70% seroprotection threshold set by the FDA, which is required to be achieved to seek an application for approval under the accelerated pathway. The company expects to start the pre-submission process with the regulatory body in second-quarter 2022.
The study will continue to monitor a subset of participants who were part of the pivotal phase III study and received VLA1553 for a period of at least five years to confirm the anticipated long-term protection offered after the administration of a single vaccine dose. Valneva is also evaluating the vaccine in a phase III study in the adolescent population, which was initiated this January.
Valneva’s shares have plunged 46.9% so far this year in comparison with the
industry
’s 11.5% decline.
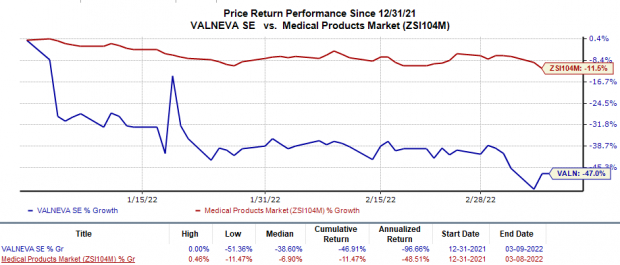
Image Source: Zacks Investment Research
The company is also set to report final lot-to-lot manufacturing consistency results — one of the standard requirements for vaccine licensure — in second-quarter 2022. VALN previously reported top-line data from the lot-to-lot phase III study for VLA1553 last December. The study met its primary endpoint demonstrating three consecutively manufactured vaccine lots elicited equivalent immune responses measured by neutralizing antibody titer GMT ratios on Day 29 after vaccination.
Chikungunya is a mosquito-borne disease caused by CHIKV. Currently, there are no effective treatments or preventive vaccines available to treat this indication. If approved, VLA1553 will also be the first vaccine for chikungunya and will be eligible for a priority review voucher. The vaccine has already been granted Breakthrough Designation therapy and Fast Track designations by the FDA.
Apart from VLA1553, the company is evaluating other vaccines for Lyme disease and COVID-19 in its pipeline.
Last October, Valneva
announced
positive top-line data from the pivotal phase III study Cov-Compare comparing Valneva’s inactivated COVID-19 vaccine, VLA2001, against
AstraZeneca
’s
AZN
COVID vaccine AZD1222. Data from the study demonstrated the superiority of VLA2001 over the AstraZeneca vaccine. The study achieved both co-primary endpoints two weeks after the second vaccination. VLA2001 produced superior neutralizing antibody titer levels than AstraZeneca’s AZD1222. Further, VLA2001 demonstrated the same effectiveness as AZD1222 in neutralizing antibody seroconversion rates above 95%. Please note that AZD1222 has been developed by AZN in partnership with the University of Oxford.
Earlier this month, VLA2001 received the first authorization for use in the Kingdom of Bahrain.
VLA15, the company’s vaccine candidate for Lyme disease is being prepared in collaboration with
Pfizer
PFE
. Last month, both PFE and VALN reported positive data from the phase II study evaluating VLA15. The study demonstrated a stronger immune response in patients who were administered three doses of the vaccine as compared to those patients who were administered two doses. Both Pfizer and Valneva plan to proceed with a three-dose primary regimen of the vaccine in a phase III study, which is expected to start in third-quarter 2022.
We remind investors that both Pfizer and Valneva entered into a collaboration in 2020 to develop and commercialize VLA15.
Zacks Rank & Another Stock to Consider
Valnevacurrently carries a Zacks Rank #2 (Buy). Another top-ranked stock in the overall healthcare sector is
BioDelivery Sciences
BDSI
, which also carries a Zacks Rank #2. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
BioDelivery Sciences’ earnings per share estimates for 2022 have increased from 33 cents to 35 cents in the past 30 days. Shares of BDSI have surged 79.7% year to date.
Earnings of BioDelivery Sciences beat estimates in three of the last four quarters and missed the mark on a single occasion, with the average surprise being 33.7%.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report









